To Be Discussed -
Atomic Physics
Atomic models
- Dalton
- J.J Thomson
- Rutherford
- Bohr
- Discovery of Neutron
Atomic Physics - Branch of Physics deals with the structure and characteristics of an atom
and subatomic particles.
Models of the Atom
- Nowadays, we know that atoms are the composition of a positively charged nucleus in the center surrounded by negatively charged electrons.
- Many attempts were made to develop atomic models to explain the structure and properties of an atom.
- Now, we are going to discuss how our modern understanding of the atom has evolved over time.
Who gives the idea of an atom
- The idea of atom was invented by two Greek philosophers, Democritus, and Leucippus in the fifth century BC.
- The Greek word ατομον (atom) means indivisible.
- They believed that atom could not be broken into smaller pieces.
Dalton's model of an atom
- In 1803, Dalton's atomic model sets up the building blocks for others to improve on.
- Though some of his conclusions were incorrect, his contributions were vital. His explanation was extra-ordinary for that period of time.
- Dalton proposed that all matters are composed of atoms
- When Dalton proposed his model electron and nucleus were unknown.
JJ Thomson's model of an atom
- JJ Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904.
- However the atomic nucleus had not been discovered yet.
- In this model, the atom is made up of negative electrons that float in a soup of positive charge, much like plums in a pudding.
- In 1906, Thomson awarded the Nobel prize for his work in this field
- There was still no understanding of how these electrons in the atom were arranged.
Rutherford's model of an atom
- In 1911, Rutherford tested Thomson's hypothesis by devising his gold foil experiment.
- His model described the atom as tiny, dense, positively charged core called nucleus surrounded by lighter, negatively charged electrons
Limitations of the Rutherford model
- An electron accelerating around the nucleus would continuously emit EM radiation and lose energy.
- It would eventually fall into the nucleus and the atom would collapse.
- However, this is not consistent with real-world observations- Atoms are stable.
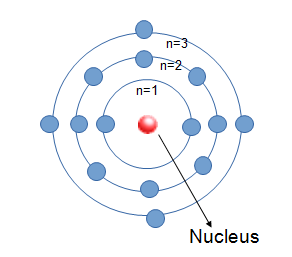
Bohr's model of an atom
- Proposed in 1913
- Revised Rutherford's atom to create a more stable model of the atom.
- Atoms are mostly empty space with a positively charged nucleus surrounded by electrons that travel in circular orbits (like solar system).
Discovery of Neutron
- In 1920, Rutherford predicted that another kind of particle must present in the nucleus along with the proton.
- Because if there were only +ve charged protons in the nucleus, then it should break into bits because of the repulsive forces between like-charged protons.
- To stays atom electrically neutral, this particle would have to be neutral itself.
- In 1932, James Chadwick discovered the neutron.
Thank You
Online Physics
Keep Learning Keep Sharing
Read Also - Love for Physics, Click below








0 Comments
If you have any doubt, please let me know